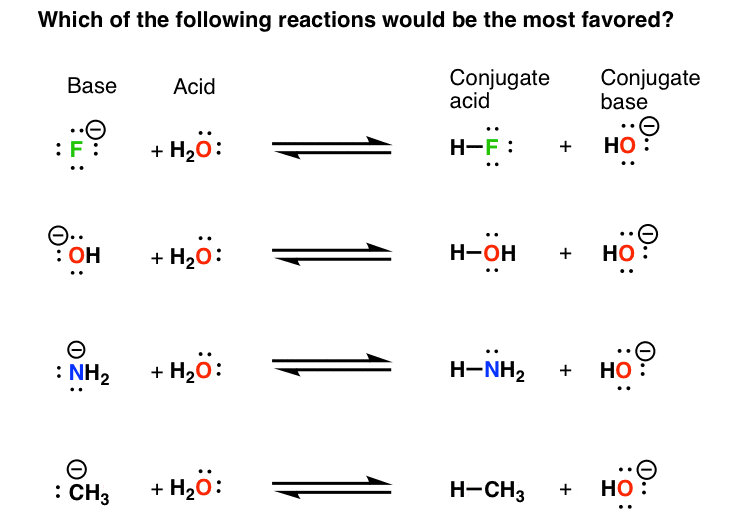
Basicity Chart
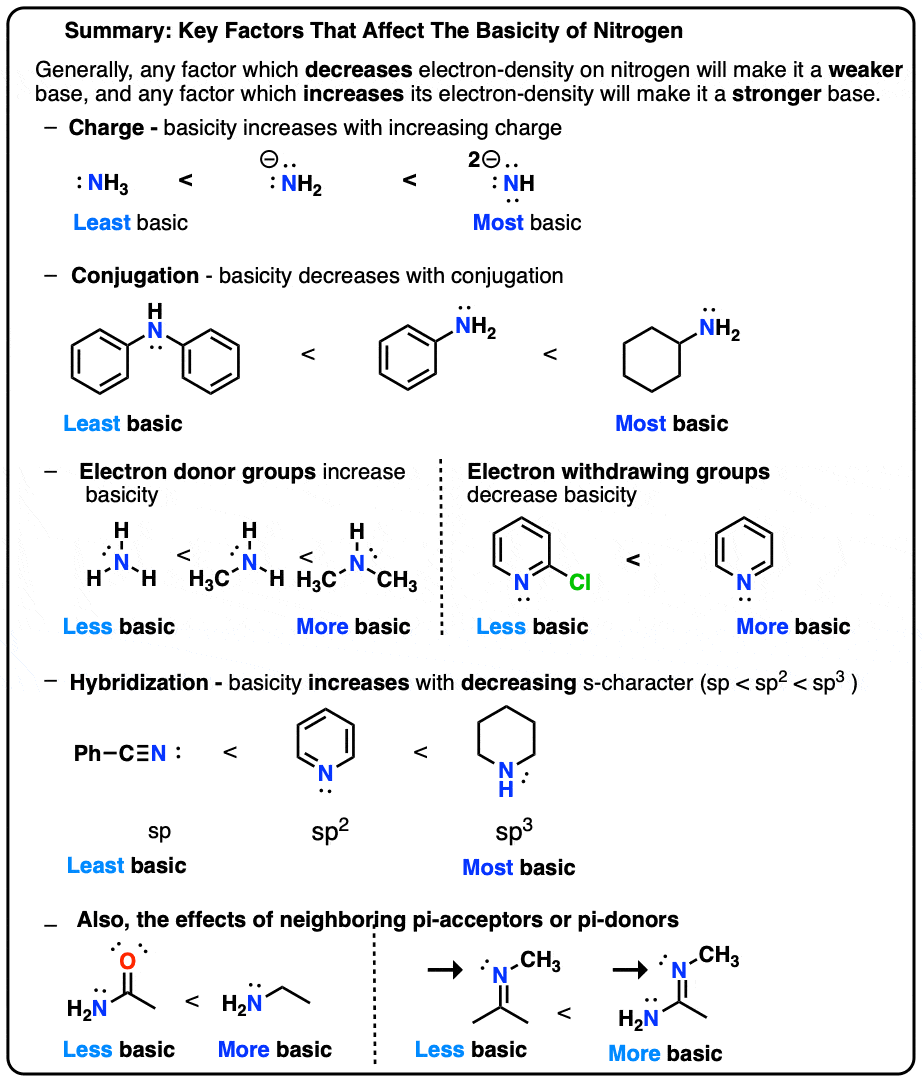
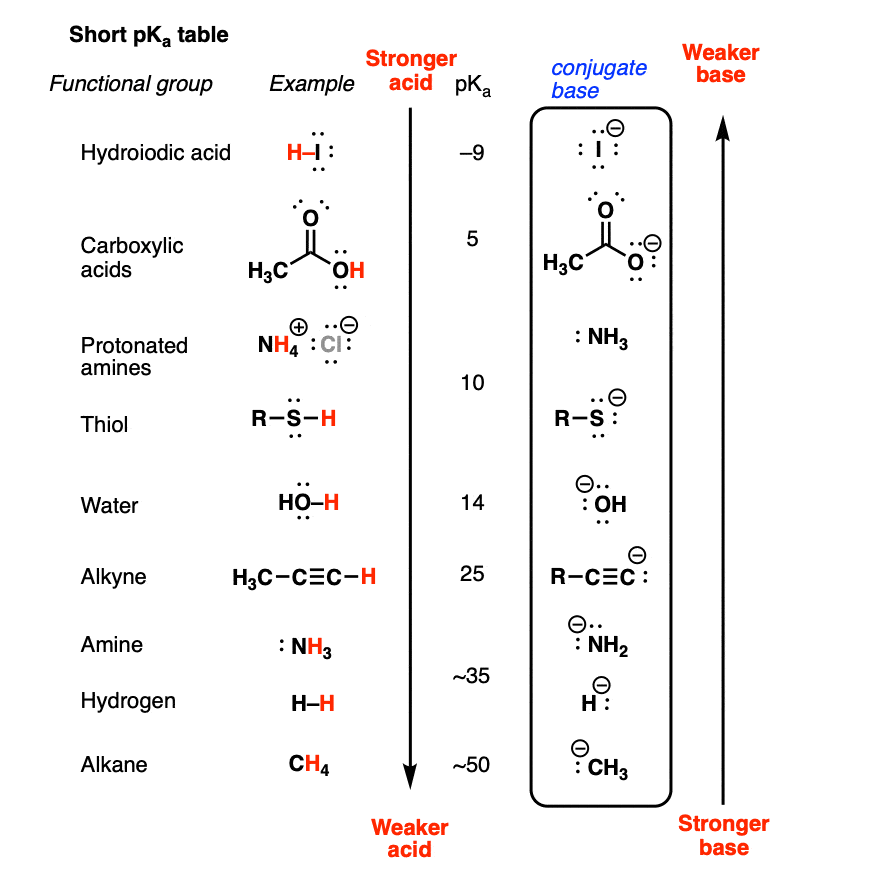
Basicity Chart - 6 realise that there is a notable drop in basicity from nitrogen to phosphorus and then a slow and continuous further diminishing. Thus, the order of basicity of aliphatic amines should be: Now i have a problem with the first line. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. I know that to compare the basic strengths, we need to find the stability of their. Basicity is how easily can the compound give up hydrogen atoms/electrons. Well, i know that b(oh)x3 b (o h) x 3 is a weak. Greater is the stability of the substituted. Acidity and basicity are experimentally determined. Basicity decreases with increasing s character basicity decreases with increasing stability, due to resonance/ delocalization (taken from this webpage). I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): The notable drop is due to the difference of the. Now i have a problem with the first line. Thus, the order of basicity of aliphatic amines should be: Greater is the stability of the substituted. While steric hindrance will decrease the rate solvent access, it more importantly also. Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3 years, 5 months ago The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. The order of acidity and basicity is based on countless experiments that establish. Well, i know that b(oh)x3 b (o h) x 3 is a weak. Acidity and basicity depend on the interaction between the acid/base and the solvent. Primary > secondary > tertiary. While steric hindrance will decrease the rate solvent access, it more importantly also. And that bihx3 b i h x 3 should have. The order of acidity and basicity is based on countless experiments that establish. Greater is the stability of the substituted. And that bihx3 b i h x 3 should have. Now i have a problem with the first line. Well, i know that b(oh)x3 b (o h) x 3 is a weak. While steric hindrance will decrease the rate solvent access, it more importantly also. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. Basicity decreases with increasing s character basicity decreases with increasing. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): I know that to compare the basic strengths, we need to find the stability of their. While steric hindrance will decrease. The first is logical, and is used to describe how basic a base is. Now i end up thinking basicity and reducing character are the same thing. Well, i know that b(oh)x3 b (o h) x 3 is a weak. 6 realise that there is a notable drop in basicity from nitrogen to phosphorus and then a slow and continuous. Well, i know that b(oh)x3 b (o h) x 3 is a weak. While steric hindrance will decrease the rate solvent access, it more importantly also. The first is logical, and is used to describe how basic a base is. Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3. While steric hindrance will decrease the rate solvent access, it more importantly also. Acidity and basicity depend on the interaction between the acid/base and the solvent. And that bihx3 b i h x 3 should have. I know that to compare the basic strengths, we need to find the stability of their. Primary > secondary > tertiary. Acidity and basicity are experimentally determined. My book defines the basicity of the acid as the number of hx+ h x + ions furnished by one mole of the acid in solution. Greater is the stability of the substituted. The first is logical, and is used to describe how basic a base is. Acidity and basicity depend on the interaction. Acidity and basicity are experimentally determined. Basicity is how easily can the compound give up hydrogen atoms/electrons. While steric hindrance will decrease the rate solvent access, it more importantly also. Order of basicity for arylamines and ammonia in gas phase ask question asked 3 years, 8 months ago modified 3 years, 5 months ago The issue is that basicity has. The order of acidity and basicity is based on countless experiments that establish. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. Basicity is how easily can the compound give up hydrogen atoms/electrons. Now i end up thinking basicity and reducing character are the same. While steric hindrance will decrease the rate solvent access, it more importantly also. Now i end up thinking basicity and reducing character are the same thing. And that bihx3 b i h x 3 should have. The order of acidity and basicity is based on countless experiments that establish. Now i have a problem with the first line. The issue is that basicity has a couple of meanings and you have to be able to tell from the context what it means. Greater is the stability of the substituted. Acidity and basicity are experimentally determined. I need to find the decreasing basicity of the following compounds(i, ii, iii, iv, respectively): Primary > secondary > tertiary. Well, i know that b(oh)x3 b (o h) x 3 is a weak. Basicity decreases with increasing s character basicity decreases with increasing stability, due to resonance/ delocalization (taken from this webpage). The first is logical, and is used to describe how basic a base is. Acidity and basicity depend on the interaction between the acid/base and the solvent. Basicity is how easily can the compound give up hydrogen atoms/electrons. 6 realise that there is a notable drop in basicity from nitrogen to phosphorus and then a slow and continuous further diminishing.Understanding basicity in organic chemistry — Master Organic Chemistry
Order of Basicity Acid and Base Strengths YouTube
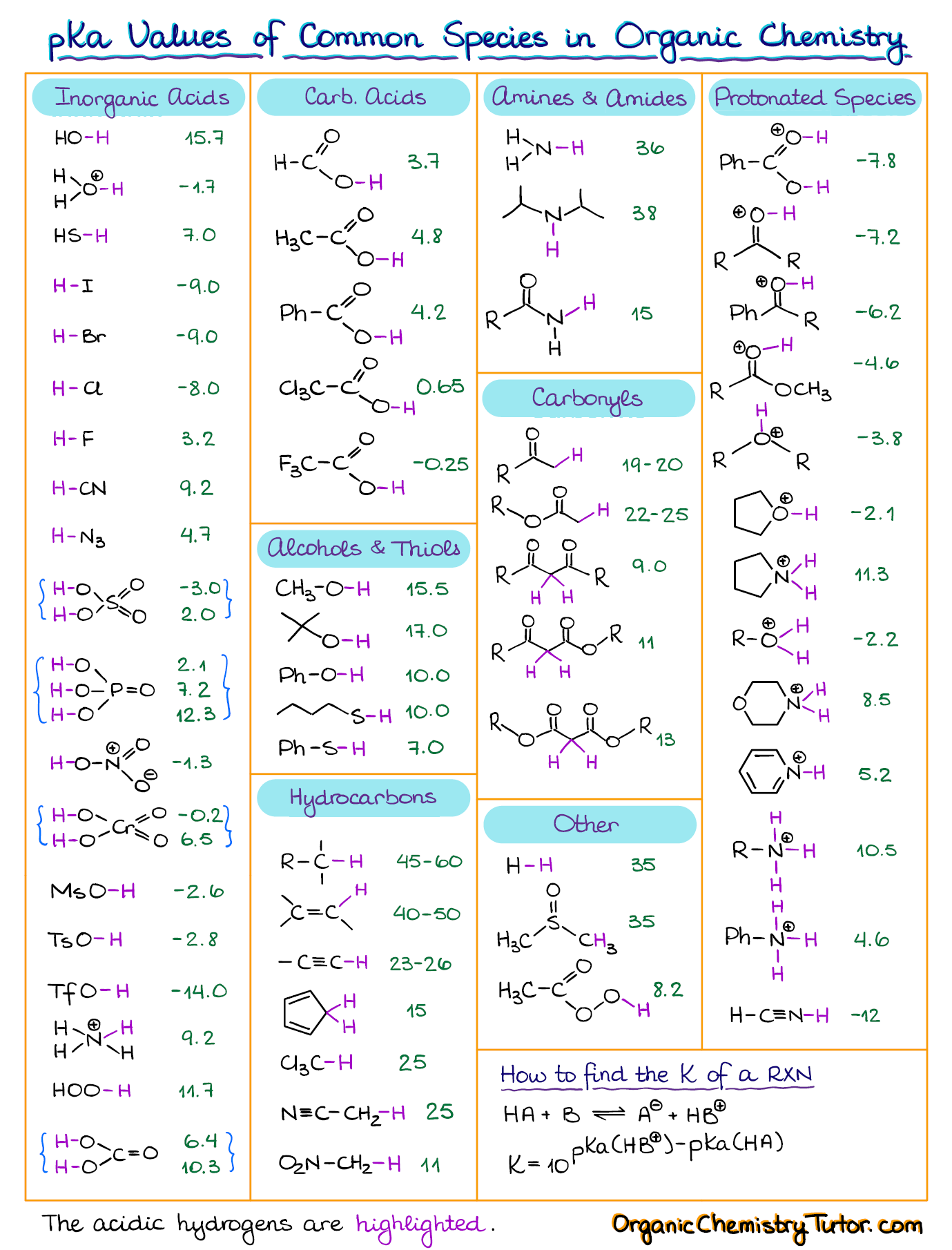
Ionization Constants of BH Onium Acids Name of Base (B) Formula of B K (BH ) pK
Acidity and Basicity Constants Acidity and basicity consta… Flickr Photo Sharing!
AcidBase Equilibrium Part 1 How to Use the pKa Table — Organic Chemistry Tutor
Secondary Amines Examples at David Masterson blog
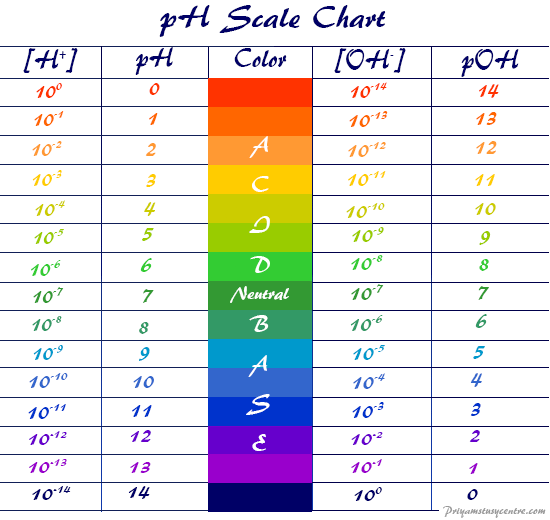
pH Scale pOH Scale Definition, Range, Chart, Measurement
Acids and Bases
Acidity, Basicity, and Stability The Relationship YouTube
Acids And Bases List Strength
I Know That To Compare The Basic Strengths, We Need To Find The Stability Of Their.
The Notable Drop Is Due To The Difference Of The.
Thus, The Order Of Basicity Of Aliphatic Amines Should Be:
My Book Defines The Basicity Of The Acid As The Number Of Hx+ H X + Ions Furnished By One Mole Of The Acid In Solution.
Related Post: