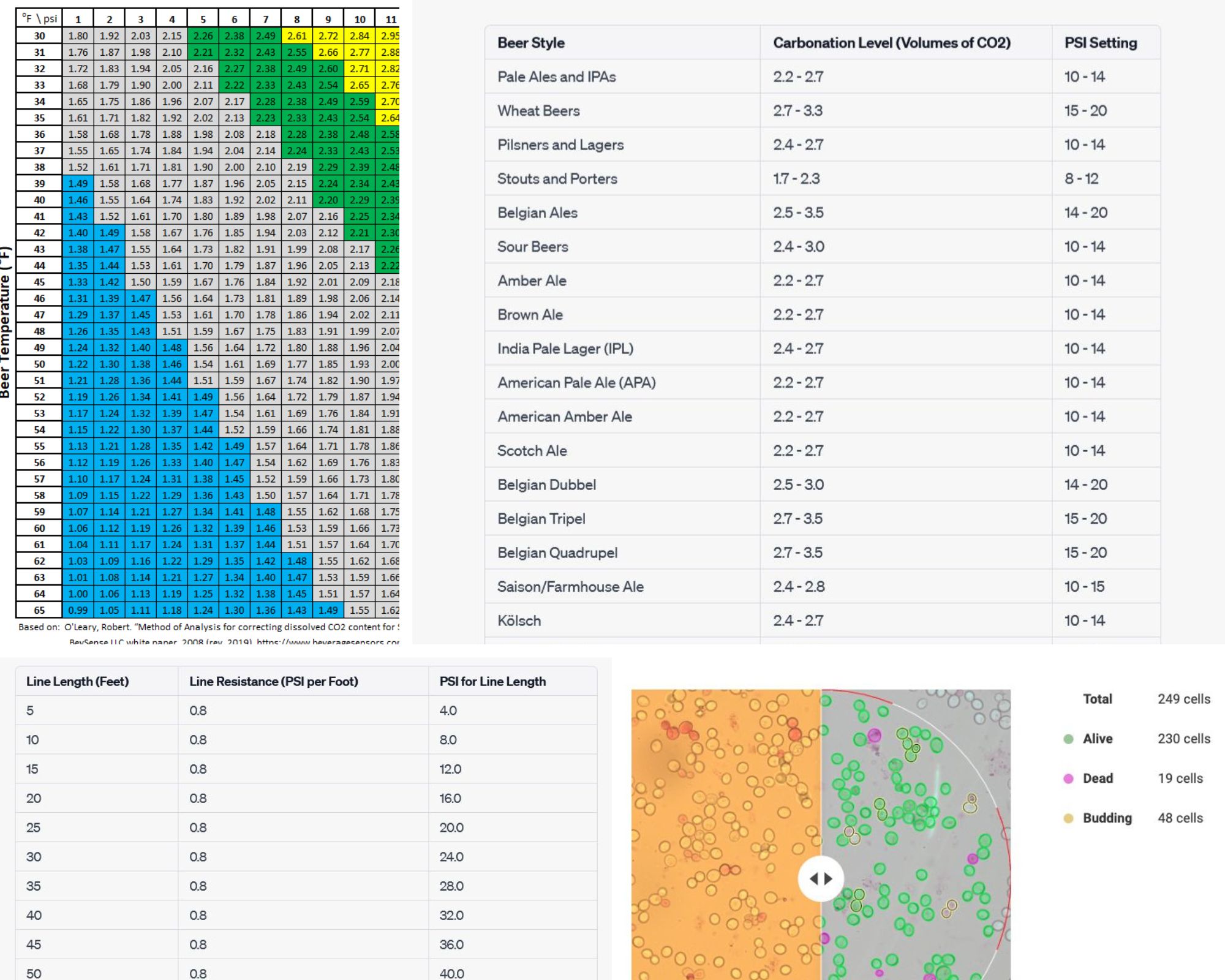
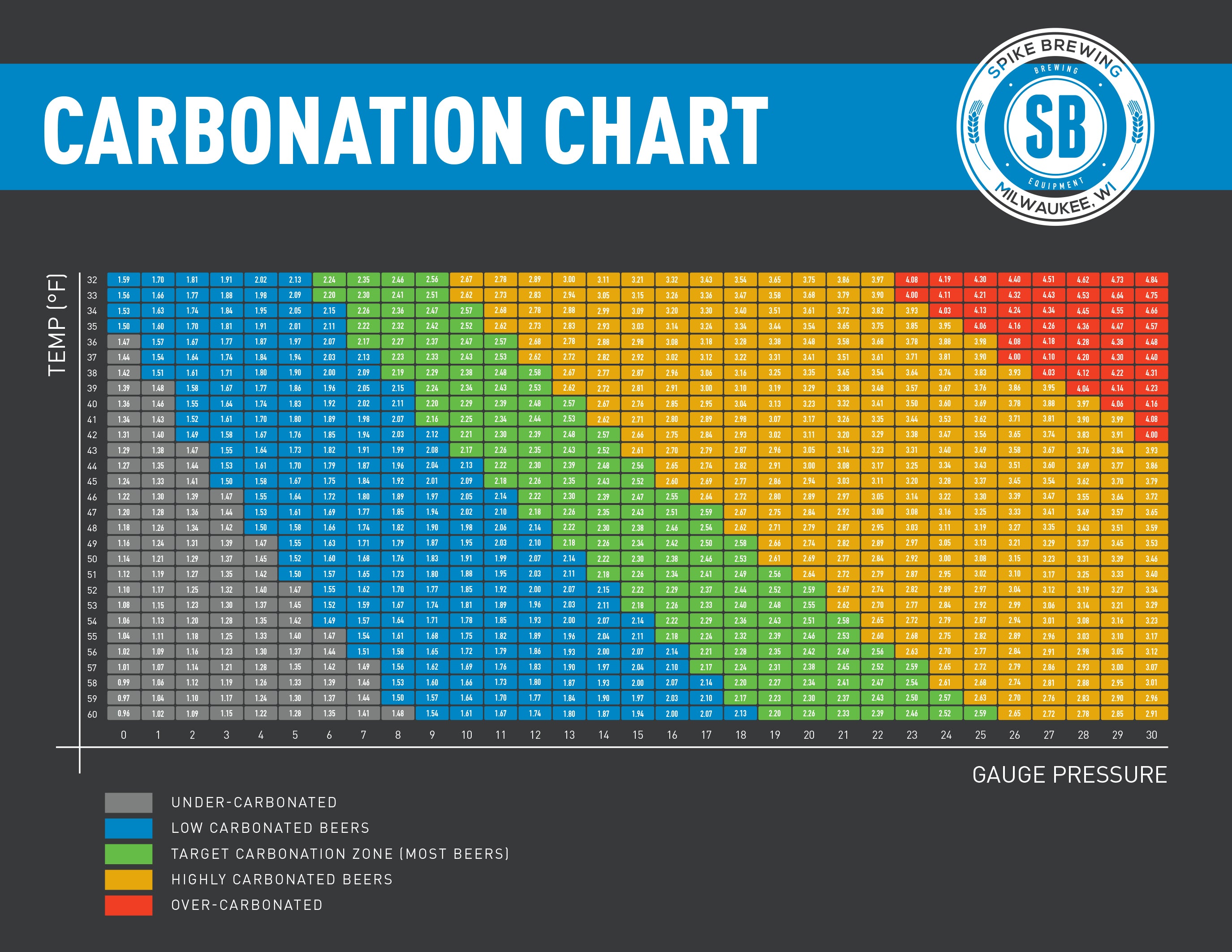
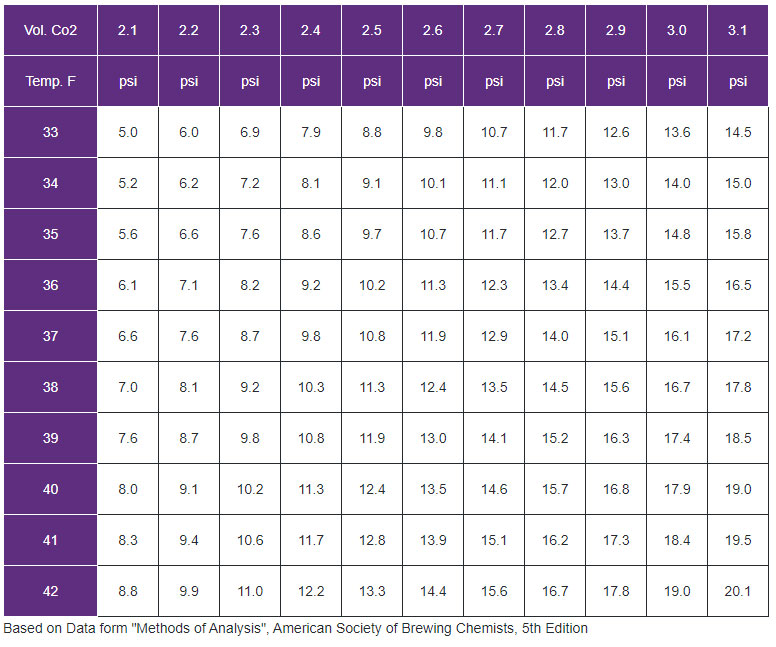
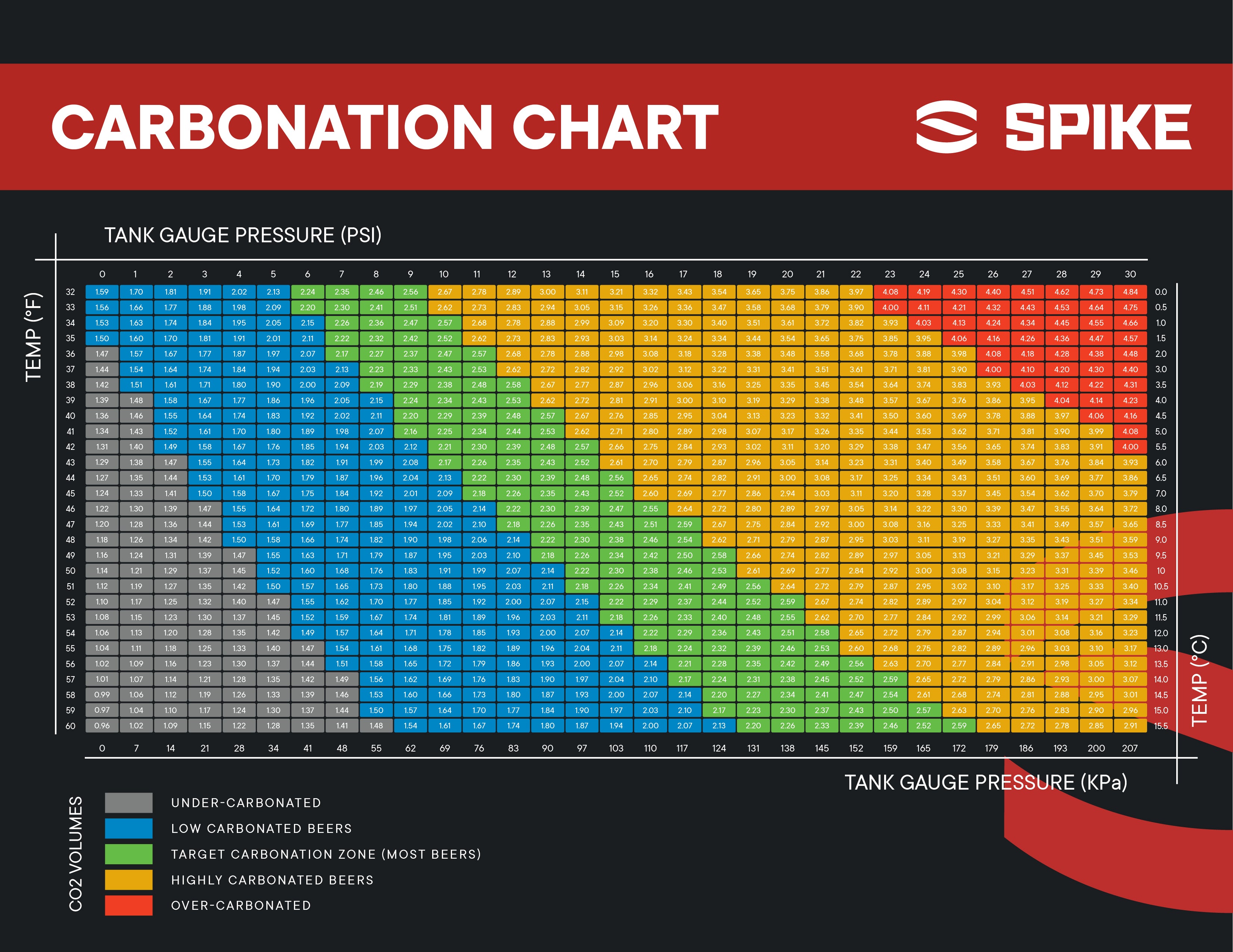
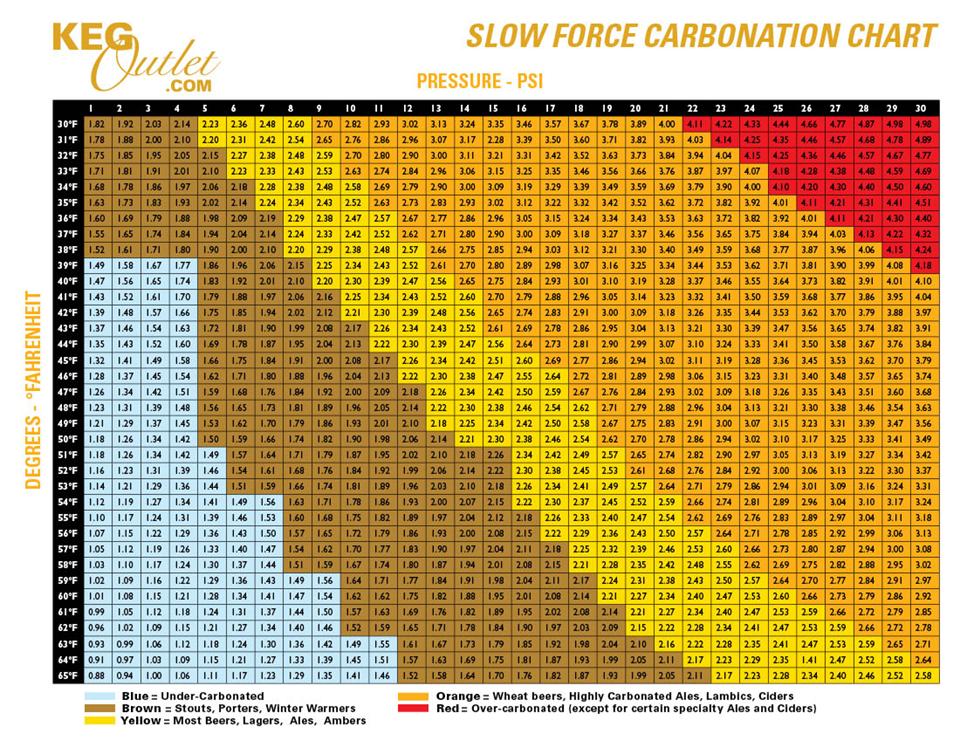
Co2 Chart For Beer
Co2 Chart For Beer - What i've tried i have very little chemistry knowledge, but. Hybridization is determined by molecular geometry. I'm getting back into home brewing after a 4 year hiatus and i am looking to do my first keezer build. Fish keepers use a table like the one below to determine dissolved $\\ce{co2}$ by observing the color of a ph indicator in water with a known concentration carbonate solution. Carbon dioxide is a linear. Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1 gj of natural gas. Water is in equilibrium with 0.78 atm n2 and possibly oversaturated by. The amount of co2 dissolved in water is proportional to the outer pressure. Carbons participating in triple bonds like those in acetylene have two regions of electron density. Could i rig up a. What i've tried i have very little chemistry knowledge, but. The first one is $$\\ce{co2 + naoh(aq). Carbons participating in triple bonds like those in acetylene have two regions of electron density. At 20°c, 1 liter water dissolves about 1.7 g co2 at normal pressure (1 atm). Hm, until co2 strips out o2 and n2, bubbles would not dissolve completely, not being co2 bubbles. I'm getting back into home brewing after a 4 year hiatus and i am looking to do my first keezer build. Carbon dioxide is a linear. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Water is in equilibrium with 0.78 atm n2 and possibly oversaturated by. Hybridization is determined by molecular geometry. I'm getting back into home brewing after a 4 year hiatus and i am looking to do my first keezer build. Water is in equilibrium with 0.78 atm n2 and possibly oversaturated by. Hybridization is determined by molecular geometry. Could i rig up a. Fish keepers use a table like the one below to determine dissolved $\\ce{co2}$ by observing the. Water is in equilibrium with 0.78 atm n2 and possibly oversaturated by. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1 gj of natural gas. Which one is the right. Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1 gj of natural gas. Co2 should be heated at room temperature so carbon becomes gaseous and oxygen becomes part of air. At 20°c, 1 liter water dissolves about 1.7 g co2 at normal pressure (1 atm). My question is about making a little jumper. Carbon dioxide is a linear. At 20°c, 1 liter water dissolves about 1.7 g co2 at normal pressure (1 atm). What the hell are you talking about? Hm, until co2 strips out o2 and n2, bubbles would not dissolve completely, not being co2 bubbles. Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1. Carbon dioxide is a linear. The first one is $$\\ce{co2 + naoh(aq). So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Hybridization is determined by molecular geometry. Which one is the right one to use? multiplying the energy of a c=o bond (from co2) by 2,. The first one is $$\\ce{co2 + naoh(aq). The amount of co2 dissolved in water is proportional to the outer pressure. What i've tried i have very little chemistry knowledge, but. Which one is the right one to use? multiplying the energy of a c=o bond (from co2) by 2, c=o bonds in co2 will have a different strength than c=o. Carbons participating in triple bonds like those in acetylene have two regions of electron density. Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1 gj of natural gas. Carbon dioxide is a linear. I'm getting back into home brewing after a 4 year hiatus and i am looking to do my first keezer. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Carbons participating in triple bonds like those in acetylene have two regions of electron density. Carbon dioxide is a linear. The first one is $$\\ce{co2 + naoh(aq). What the hell are you talking about? Carbons participating in triple bonds like those in acetylene have two regions of electron density. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Which one is the right one to use? multiplying the energy of a c=o bond (from co2) by 2, c=o bonds in. Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1 gj of natural gas. Which one is the right one to use? multiplying the energy of a c=o bond (from co2) by 2, c=o bonds in co2 will have a different strength than c=o bonds in other. At 20°c, 1 liter water dissolves about. My question is about making a little jumper co2 line. What the hell are you talking about? Background i am trying to determine how many kg of $\\ce{co2}$ are released when burning 1 gj of natural gas. Co2 should be heated at room temperature so carbon becomes gaseous and oxygen becomes part of air. Carbon dioxide is a linear. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. At 20°c, 1 liter water dissolves about 1.7 g co2 at normal pressure (1 atm). Hm, until co2 strips out o2 and n2, bubbles would not dissolve completely, not being co2 bubbles. I'm getting back into home brewing after a 4 year hiatus and i am looking to do my first keezer build. Could i rig up a. Water is in equilibrium with 0.78 atm n2 and possibly oversaturated by. Which one is the right one to use? multiplying the energy of a c=o bond (from co2) by 2, c=o bonds in co2 will have a different strength than c=o bonds in other. Hybridization is determined by molecular geometry. The amount of co2 dissolved in water is proportional to the outer pressure.Carbonation Chart For Beer A Complete Guide for Brewers
Carbonation Priming Chart Brew Your Own
Carbonation Chart Spike Brewing
Better Bottling Craft Beer & Brewing
CO2 Pressure to Carb Coffee Porter Make Beer at Home Forums Brewer's Friend
Beer Carbonation Chart The Importance of PSI DrinkTanks®
Kegerator Basics What You Need To Know About CO2
Carbonation Chart Poster Spike Brewing
Keg Carbonation Chart
The Definitive Guide to Force Carbonating Your Beer
What I've Tried I Have Very Little Chemistry Knowledge, But.
Fish Keepers Use A Table Like The One Below To Determine Dissolved $\\Ce{Co2}$ By Observing The Color Of A Ph Indicator In Water With A Known Concentration Carbonate Solution.
Carbons Participating In Triple Bonds Like Those In Acetylene Have Two Regions Of Electron Density.
The First One Is $$\\Ce{Co2 + Naoh(Aq).
Related Post: