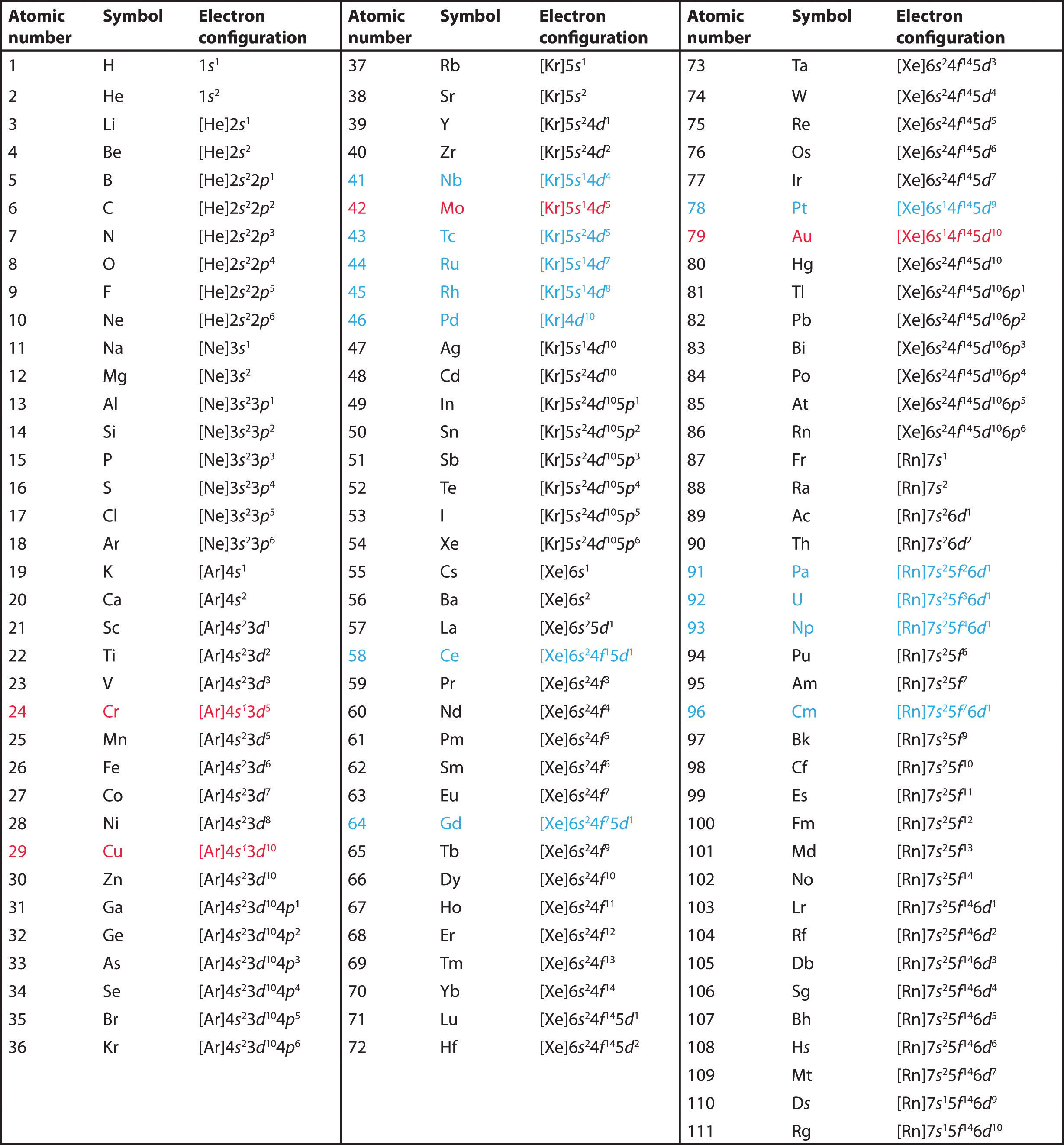
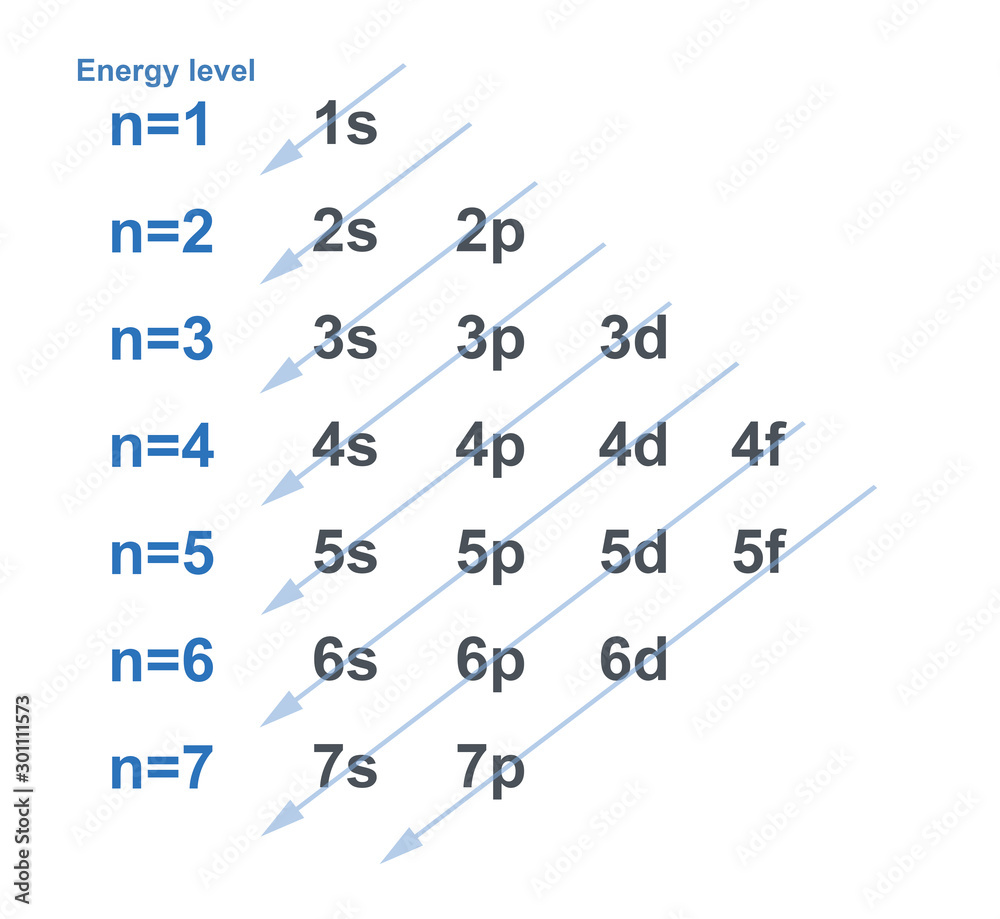
Electron Configuration Chart
Electron Configuration Chart - On the other hand, an element located in group 17 has 7 valence electrons, which implies that it can complete its octet by gaining 1 electron. Valence is a term that deals with the electrons that are most typically involved in the bonding characteristics of an atom. Besides that, when 1 4l(l + 1) is small compared to s(s + 1), μs+l ≈ μs is a good approximation. These electrons are found in the s and p orbitals of the. Neon is a noble gas. That is when we have a light. Lithium has an electronic configuration of 1s^2. Well, we just need to add all the powers of the numbers to get the total number of electrons, and since they are all neutral atoms, that will also be their proton number, which. Electronegativity values show the relative attraction for valence electrons that each element or atom has. The valency of lithium is predicated on the relationships shown on the periodic table but the electron configuration will work. Electronegativity values show the relative attraction for valence electrons that each element or atom has. Well, we just need to add all the powers of the numbers to get the total number of electrons, and since they are all neutral atoms, that will also be their proton number, which. When that happens, the atom will. Put another way, the number of electrons that can be put in any shell is 2n2 so, for the n=1 shell you get 2 ×12 or 2. That is when we have a light. Beryllium (be) has the atomic number 4 (not 2), so its electron configuration is 1s22s2. On the other hand, an element located in group 17 has 7 valence electrons, which implies that it can complete its octet by gaining 1 electron. Lithium has an electronic configuration of 1s^2. Besides that, when 1 4l(l + 1) is small compared to s(s + 1), μs+l ≈ μs is a good approximation. The valency of lithium is predicated on the relationships shown on the periodic table but the electron configuration will work. On the other hand, an element located in group 17 has 7 valence electrons, which implies that it can complete its octet by gaining 1 electron. Valence is a term that deals with the electrons that are most typically involved in the bonding characteristics of an atom. Electronegativity values show the relative attraction for valence electrons that each element or. On the other hand, an element located in group 17 has 7 valence electrons, which implies that it can complete its octet by gaining 1 electron. The valency of lithium is predicated on the relationships shown on the periodic table but the electron configuration will work. That is when we have a light. Put another way, the number of electrons. These electrons are found in the s and p orbitals of the. When that happens, the atom will. Electronegativity values show the relative attraction for valence electrons that each element or atom has. The noble gasses all have filled outer electron shells. That is when we have a light. These electrons are found in the s and p orbitals of the. Neon is a noble gas. Beryllium (be) has the atomic number 4 (not 2), so its electron configuration is 1s22s2. Fluorine has the highest electronegativity with a value of 4. Electronegativity values show the relative attraction for valence electrons that each element or atom has. 6 given that n (your principle quantum number) is 3, and s (your angular quantum number) is 1, we can make a couple of statements right off the bat: Besides that, when 1 4l(l + 1) is small compared to s(s + 1), μs+l ≈ μs is a good approximation. Electronegativity values show the relative attraction for valence electrons that. He has the atomic number 2, so its electron configuration is 1s2. Put another way, the number of electrons that can be put in any shell is 2n2 so, for the n=1 shell you get 2 ×12 or 2. Lithium has an electronic configuration of 1s^2. These electrons are found in the s and p orbitals of the. The valency. He has the atomic number 2, so its electron configuration is 1s2. The noble gasses all have filled outer electron shells. Neon is a noble gas. The valency of lithium is predicated on the relationships shown on the periodic table but the electron configuration will work. 6 given that n (your principle quantum number) is 3, and s (your angular. When that happens, the atom will. Besides that, when 1 4l(l + 1) is small compared to s(s + 1), μs+l ≈ μs is a good approximation. Electronegativity values show the relative attraction for valence electrons that each element or atom has. Valence is a term that deals with the electrons that are most typically involved in the bonding characteristics. Besides that, when 1 4l(l + 1) is small compared to s(s + 1), μs+l ≈ μs is a good approximation. 6 given that n (your principle quantum number) is 3, and s (your angular quantum number) is 1, we can make a couple of statements right off the bat: The noble gasses all have filled outer electron shells. Valence. 6 given that n (your principle quantum number) is 3, and s (your angular quantum number) is 1, we can make a couple of statements right off the bat: Valence is a term that deals with the electrons that are most typically involved in the bonding characteristics of an atom. Well, we just need to add all the powers of. When that happens, the atom will. Electronegativity values show the relative attraction for valence electrons that each element or atom has. These electrons are found in the s and p orbitals of the. Valence is a term that deals with the electrons that are most typically involved in the bonding characteristics of an atom. Put another way, the number of electrons that can be put in any shell is 2n2 so, for the n=1 shell you get 2 ×12 or 2. He has the atomic number 2, so its electron configuration is 1s2. Besides that, when 1 4l(l + 1) is small compared to s(s + 1), μs+l ≈ μs is a good approximation. Beryllium (be) has the atomic number 4 (not 2), so its electron configuration is 1s22s2. The valency of lithium is predicated on the relationships shown on the periodic table but the electron configuration will work. Neon is a noble gas. The noble gasses all have filled outer electron shells. That is when we have a light. Fluorine has the highest electronegativity with a value of 4.Electron Configuration
2.4 Electron Configurations Chemistry LibreTexts
2.2 Electron Configurations Chemistry LibreTexts
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Trends ALevel
List of Electron Configuration Chart of All Elements [PDF]
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts
Get the Detailed Periodic table (With Electron Configuration)
List of Electron Configurations of Elements
Electron Configuration Chart
Electronic configuration Definition, Orbitals, & Facts Britannica
Well, We Just Need To Add All The Powers Of The Numbers To Get The Total Number Of Electrons, And Since They Are All Neutral Atoms, That Will Also Be Their Proton Number, Which.
6 Given That N (Your Principle Quantum Number) Is 3, And S (Your Angular Quantum Number) Is 1, We Can Make A Couple Of Statements Right Off The Bat:
Lithium Has An Electronic Configuration Of 1S^2.
On The Other Hand, An Element Located In Group 17 Has 7 Valence Electrons, Which Implies That It Can Complete Its Octet By Gaining 1 Electron.
Related Post:
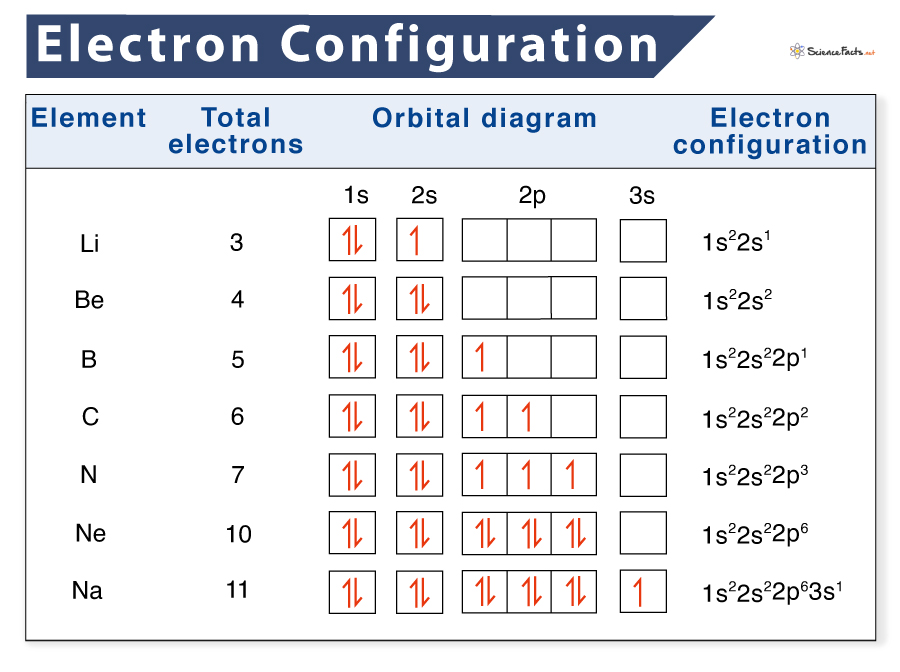


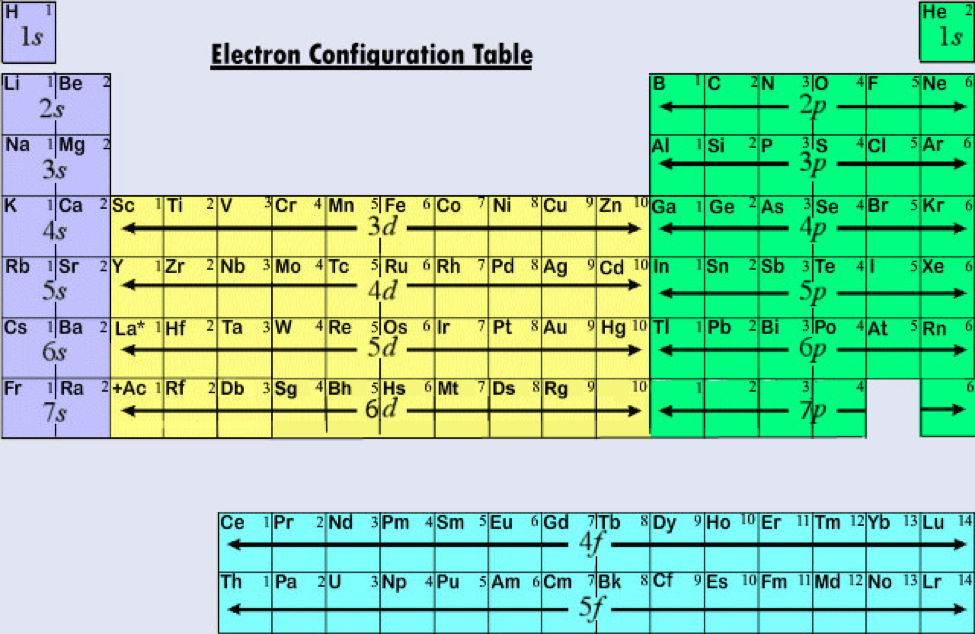
![List of Electron Configuration Chart of All Elements [PDF]](https://iperiodictable.com/wp-content/uploads/2021/03/What-is-Electron-Configuration.png?6bfec1&6bfec1)