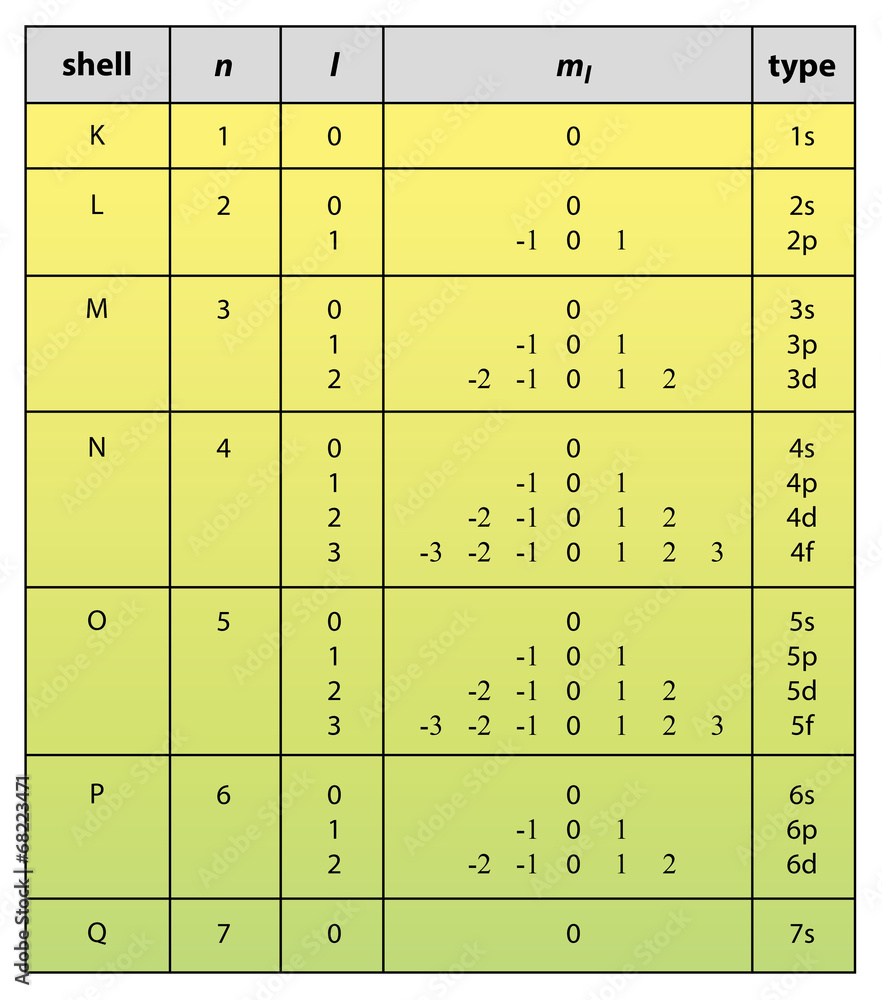
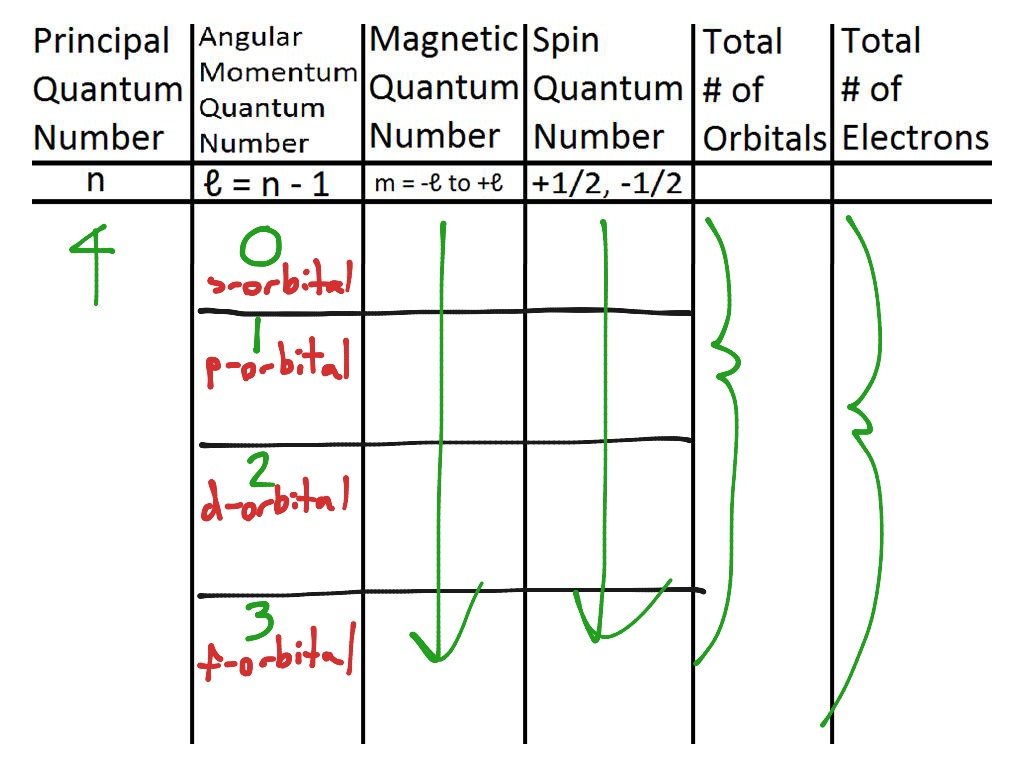
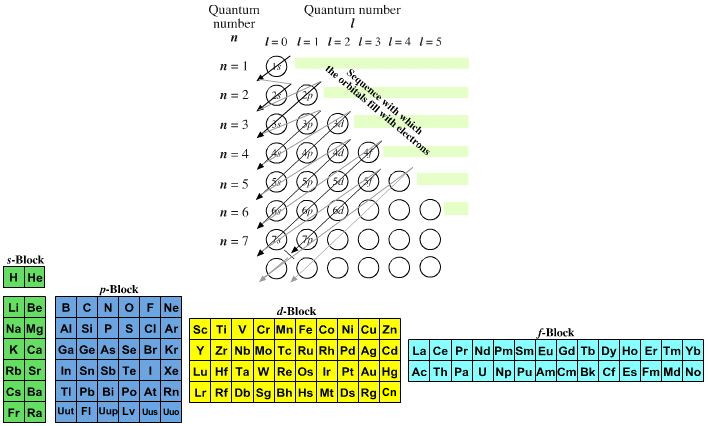
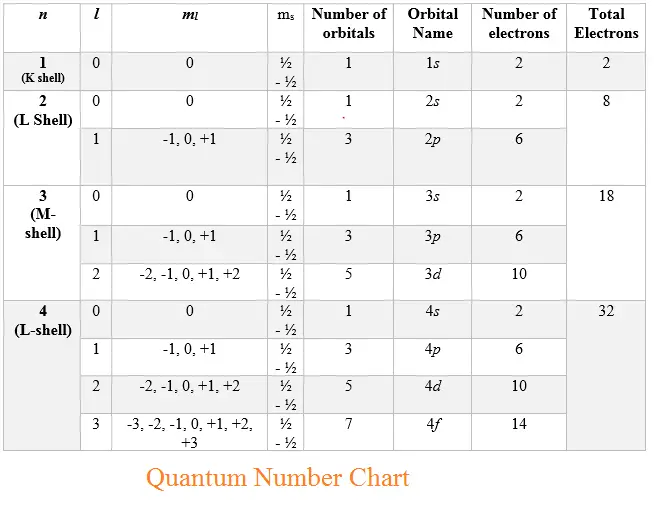
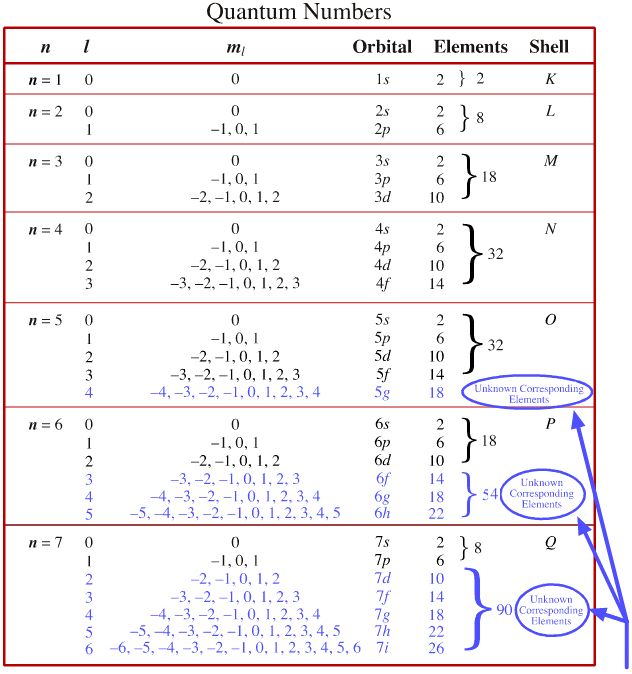
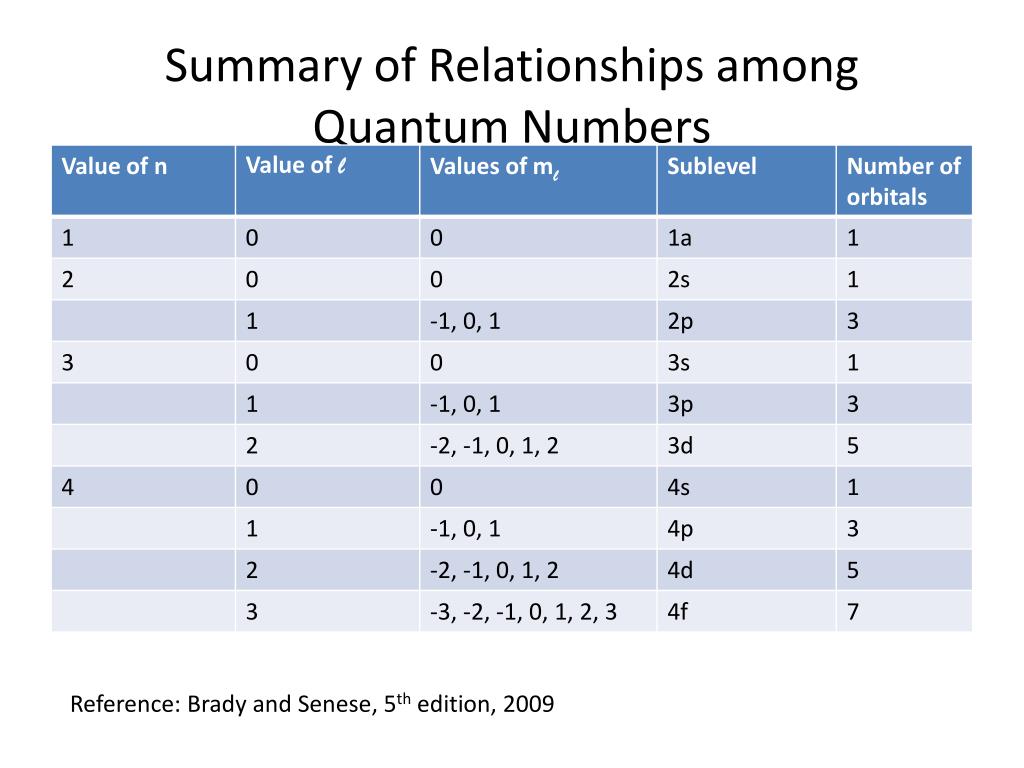
Quantum Number Chart
Quantum Number Chart - It is not limited to the first row. Prime numbers aren't divisible by anything except 1 and itself, composite numbers are all real numbers that aren't prime. So, for a system you can have certain observables, for example considering a single particle a system, it's position. Percent or % means out of 100 or per 100, therefore 5% can be written as 5 100. The bohr's atomic model although assumes that the energy of the electron depends only on the principle quantum number (n) of the orbit. The principal quantum number #n# = 5 and the azimuthal quantum number #l# = 1 specify a 5p orbital. Many transition metals have variable oxidation states. As you know, four quantum numbers are. When dealing with percents the word of means times or to multiply. Put another way, the possible values of the four quantum numbers account for the fact that the second shell can accommodate eight electrons and the 2p subshell can hold two electrons in. The bohr's atomic model although assumes that the energy of the electron depends only on the principle quantum number (n) of the orbit. As you know, four quantum numbers are. The principal quantum number #n# = 5 and the azimuthal quantum number #l# = 1 specify a 5p orbital. When dealing with percents the word of means times or to multiply. Put another way, the possible values of the four quantum numbers account for the fact that the second shell can accommodate eight electrons and the 2p subshell can hold two electrons in. Prime numbers are divisible only by 1 and itself, i.e. Percent or % means out of 100 or per 100, therefore 5% can be written as 5 100. It is not limited to the first row. So, for a system you can have certain observables, for example considering a single particle a system, it's position. Prime numbers aren't divisible by anything except 1 and itself, composite numbers are all real numbers that aren't prime. Prime numbers are divisible only by 1 and itself, i.e. Put another way, the possible values of the four quantum numbers account for the fact that the second shell can accommodate eight electrons and the 2p subshell can hold two electrons in. So, for a system you can have certain observables, for example considering a single particle a system, it's. Prime numbers aren't divisible by anything except 1 and itself, composite numbers are all real numbers that aren't prime. The principal quantum number #n# = 5 and the azimuthal quantum number #l# = 1 specify a 5p orbital. I assume you are asking the question in context of quantum or statistical mechanics. When dealing with percents the word of means. Many transition metals have variable oxidation states. According to the quantum theory, the vacuum contains virtual particles which are in a continuous state of fluctuation.casimir realised that between two plates, only those virtual photons whose. Prime numbers are divisible only by 1 and itself, i.e. I assume you are asking the question in context of quantum or statistical mechanics. When. Many transition metals have variable oxidation states. The principal quantum number #n# = 5 and the azimuthal quantum number #l# = 1 specify a 5p orbital. Put another way, the possible values of the four quantum numbers account for the fact that the second shell can accommodate eight electrons and the 2p subshell can hold two electrons in. Prime numbers. Prime numbers are divisible only by 1 and itself, i.e. This assumption is flawed and was further. The bohr's atomic model although assumes that the energy of the electron depends only on the principle quantum number (n) of the orbit. Many transition metals have variable oxidation states. Prime numbers aren't divisible by anything except 1 and itself, composite numbers are. Put another way, the possible values of the four quantum numbers account for the fact that the second shell can accommodate eight electrons and the 2p subshell can hold two electrons in. This assumption is flawed and was further. As you know, four quantum numbers are. Many transition metals have variable oxidation states. According to the quantum theory, the vacuum. It is not limited to the first row. Prime numbers are divisible only by 1 and itself, i.e. Many transition metals have variable oxidation states. So, for a system you can have certain observables, for example considering a single particle a system, it's position. I assume you are asking the question in context of quantum or statistical mechanics. The principal quantum number #n# = 5 and the azimuthal quantum number #l# = 1 specify a 5p orbital. When dealing with percents the word of means times or to multiply. This assumption is flawed and was further. As you know, four quantum numbers are. Percent or % means out of 100 or per 100, therefore 5% can be written. Prime numbers aren't divisible by anything except 1 and itself, composite numbers are all real numbers that aren't prime. According to the quantum theory, the vacuum contains virtual particles which are in a continuous state of fluctuation.casimir realised that between two plates, only those virtual photons whose. When dealing with percents the word of means times or to multiply. The. It is not limited to the first row. According to the quantum theory, the vacuum contains virtual particles which are in a continuous state of fluctuation.casimir realised that between two plates, only those virtual photons whose. Many transition metals have variable oxidation states. This assumption is flawed and was further. When dealing with percents the word of means times or. The bohr's atomic model although assumes that the energy of the electron depends only on the principle quantum number (n) of the orbit. So, for a system you can have certain observables, for example considering a single particle a system, it's position. Prime numbers are divisible only by 1 and itself, i.e. This assumption is flawed and was further. It is not limited to the first row. I assume you are asking the question in context of quantum or statistical mechanics. Prime numbers aren't divisible by anything except 1 and itself, composite numbers are all real numbers that aren't prime. Many transition metals have variable oxidation states. According to the quantum theory, the vacuum contains virtual particles which are in a continuous state of fluctuation.casimir realised that between two plates, only those virtual photons whose. Put another way, the possible values of the four quantum numbers account for the fact that the second shell can accommodate eight electrons and the 2p subshell can hold two electrons in. As you know, four quantum numbers are.Quantum Number Definition Types Chart And Quiz
Quantum Numbers Chart
Quantum Number Orbitals Diagram, Definition, Chart, Shape
Quantum Numbers Chart Chemistry
Principal Quantum Number Chart
Quantum Numbers Chart physicscatalyst's Blog
Quantum Number Definition Types Chart And Quiz
Principal Quantum Number Chart
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS
PPT Principle Quantum Numbers PowerPoint Presentation, free download ID5519904
The Principal Quantum Number #N# = 5 And The Azimuthal Quantum Number #L# = 1 Specify A 5P Orbital.
When Dealing With Percents The Word Of Means Times Or To Multiply.
Percent Or % Means Out Of 100 Or Per 100, Therefore 5% Can Be Written As 5 100.
Related Post: