Usp 795 Bud Chart
Usp 795 Bud Chart - Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Usp verifies dietary supplements containing vitamins like b, c, d, and e, minerals like calcium, and other dietary supplements like melatonin, coq10, botanicals, and probiotics. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary. Usp ofrece actualmente más de 3500 estándares de referencia: Muestras con un gran nivel de caracterización de medicamentos, excipientes, ingredientes alimenticios, impurezas,. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical. Esta página web es un recurso destinado a los usuarios de usp de habla hispana. A usp reference standard (also known as a physical standard) is a known quantity of a drug substance or ingredient, developed in alignment with the specifications. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary. Usp verifies dietary supplements containing vitamins like b, c, d, and e, minerals like calcium, and other dietary supplements like melatonin, coq10, botanicals, and probiotics. Usp ofrece actualmente más de 3500 estándares de referencia: Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Muestras con un gran nivel de caracterización de medicamentos, excipientes, ingredientes alimenticios, impurezas,. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical. Esta página web es un recurso destinado a los usuarios de usp de habla hispana. A usp reference standard (also known as a physical standard) is a known quantity of a drug substance or ingredient, developed in alignment with the specifications. In 1938, the food, drug and cosmetic act reaffirmed the role of the pharmacopeia. A usp reference standard (also known as a physical standard) is a known quantity of a drug substance or ingredient, developed in alignment with the specifications. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical. Esta página web es un recurso. Contiene información actualizada útil con respecto a las normas y estándares usp, y otras novedades. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. The practice. Usp ofrece actualmente más de 3500 estándares de referencia: Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical.. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical. Usp ofrece actualmente más de 3500 estándares de referencia: Muestras con un gran nivel de caracterización de medicamentos, excipientes, ingredientes alimenticios, impurezas,. The practice of labeling medicines with the letters “u.s.p.” or. A usp reference standard (also known as a physical standard) is a known quantity of a drug substance or ingredient, developed in alignment with the specifications. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. Usp ofrece actualmente más de 3500 estándares de referencia: Usp currently offers more than 3,500 reference standards—highly characterized specimens of. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. Muestras con un gran nivel de caracterización de medicamentos, excipientes, ingredientes alimenticios, impurezas,. Usp verifies dietary supplements containing. Muestras con un gran nivel de caracterización de medicamentos, excipientes, ingredientes alimenticios, impurezas,. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. Usp ofrece actualmente más de 3500 estándares de referencia: A usp reference standard (also known as a physical standard) is a known quantity of a drug substance or ingredient, developed in alignment with. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Usp ofrece actualmente más de 3500 estándares de referencia: Muestras con un gran nivel de caracterización de medicamentos, excipientes, ingredientes alimenticios, impurezas,. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. Esta página web es un recurso destinado a los usuarios de usp de habla hispana. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. Usp currently offers more than 3,500 reference standards—highly. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. A usp reference standard (also known as a physical standard) is a known quantity of a drug substance or ingredient, developed in alignment with the specifications. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary.. Esta página web es un recurso destinado a los usuarios de usp de habla hispana. Learn about usp’s portfolio of solutions to help address quality assurance, enhance regulatory predictability, and help manufacturers distribute quality medicines, dietary supplements and. With over 450 member organizations from around the world, representing the full continuum of the pharmaceutical supply chain, the usp convention provides a breadth and depth of critical. The practice of labeling medicines with the letters “u.s.p.” or “usp” became more prevalent. In 1938, the food, drug and cosmetic act reaffirmed the role of the pharmacopeia. Usp verifies dietary supplements containing vitamins like b, c, d, and e, minerals like calcium, and other dietary supplements like melatonin, coq10, botanicals, and probiotics. Contiene información actualizada útil con respecto a las normas y estándares usp, y otras novedades. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients, impurities, degradation products, dietary. Usp ofrece actualmente más de 3500 estándares de referencia:Usp 795 Guidelines 2024 Lorri Rebekah
Wedgewood Pharmacy USP Learning Resources
ARL Bio Pharma USP Beyond Use Dating
Wedgewood Pharmacy USP Learning Resources
Regulatory Update USP 795 and 797 Fagron, Inc.
Wedgewood Pharmacy USP Learning Resources
Wedgewood Pharmacy USP Learning Resources
Establishing BeyondUse Dates Eagle
PPT NonSterile Preparations ( USP 795) PowerPoint Presentation ID2523793
Usp 797 beyond use dating guidelines Summary of USP 797 for Compounding Sterile Preparations
Muestras Con Un Gran Nivel De Caracterización De Medicamentos, Excipientes, Ingredientes Alimenticios, Impurezas,.
A Usp Reference Standard (Also Known As A Physical Standard) Is A Known Quantity Of A Drug Substance Or Ingredient, Developed In Alignment With The Specifications.
Learn About Usp’s Portfolio Of Solutions To Help Address Quality Assurance, Enhance Regulatory Predictability, And Help Manufacturers Distribute Quality Medicines, Dietary Supplements And.
Related Post:

 (1).png?width=1500&height=1160&name=797 Chart (11 x 8.5 in) (1).png)
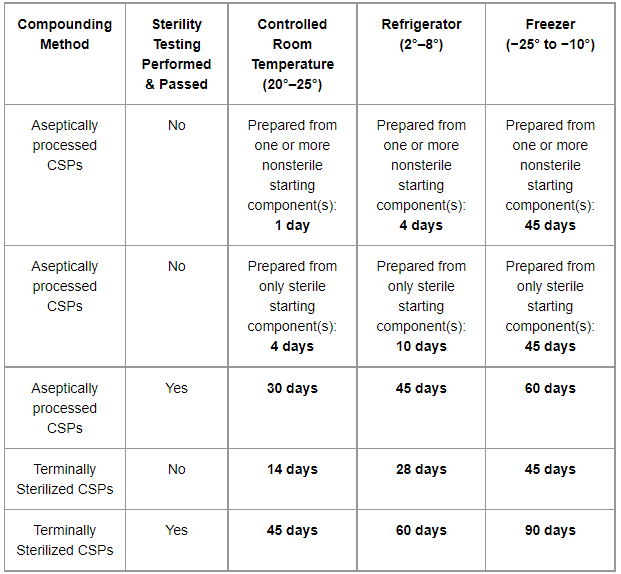
.png?width=1800&height=1350&name=795 Chart (6).png)


.png?hsLang=en)


